Reliable Testing for Montana Medicinal Cannabis
Stillwater Labs is Montana’s oldest and most established medical marijuana testing laboratory. We have spent years developing a state-of-the-art testing and diagnostic laboratory nestled in the heart of the Flathead Valley. Our team of analytical scientists and couriers can help medical marijuana producers of any size test for compliance, research, and development.


Test Your Products
Quickly and Easily with Stillwater
Order Tests
Arrange a Pick-up
Get Results
Stay Compliant
with the Right Tests
Free Terpene Analysis
Check out our long term testing trends of cannabis strains
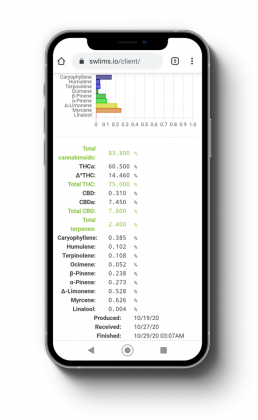
Testing Information
At Your Fingertips
Stillwater Laboratories utilizes a custom Lab Inventory Management system that allows our clients to access all of your testing information, pending testing results, and orders all at the click of a button. Our LIM system also allows clients to compare your test results and easily export all the data in an easily accessible format. Users can even view how your results compare to the rest of the industry testing data and easily make corrections without having to guess.
The Stillwater LIM system is the only testing software that is purpose-built for Montanans by Montanans, and that has allowed us to customize our approach to testing by streamlining our process making the fastest test turnarounds possible in Montana.